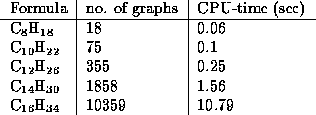Next: The use of
Up: MOLGEN, a generator of...
Previous: The computation of
MOLGEN is designed both for educational purposes and for research. In
chemical education it helps to illustrate the vast amount of combinatorial and
geometric possibilities to connect atoms in a prescribed way to form a molecule.
The student can enter a chemical formula together with
valences of the occurring atoms and then he will, first of all, be shown if
there exist any molecular graphs corresponding to the given brutto formula
and valences at all.
The next information he gets is, how many such molecular graphs there
are (if there are any, and if the computer can manage the problem in question in
reasonable time).
For example, if he enters C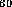 , prescribes valence 3 for every carbon
atom (since in fact C
, prescribes valence 3 for every carbon
atom (since in fact C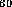 H
H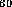 is meant), together with 12 fivemembered
rings of carbon atoms as macro-atoms, the generator yields 7848 simple graphs
consisting of 12 points (=macro-atoms) of valence 5. In the next step of
expanding the macro-atoms
MOLGEN starts generation (since the
total number of atoms is less than 100), but the generated number of molecular
graphs will be more or less infinite, even if there are 10
nonoverlapping benzene rings prescribed as macroatoms.
More moderate questions show that there are
altogether 217 molecular graphs for C
is meant), together with 12 fivemembered
rings of carbon atoms as macro-atoms, the generator yields 7848 simple graphs
consisting of 12 points (=macro-atoms) of valence 5. In the next step of
expanding the macro-atoms
MOLGEN starts generation (since the
total number of atoms is less than 100), but the generated number of molecular
graphs will be more or less infinite, even if there are 10
nonoverlapping benzene rings prescribed as macroatoms.
More moderate questions show that there are
altogether 217 molecular graphs for C H
H that there are exactly
22 dioxines, i.e. molecular graphs with the chemical formula
C
that there are exactly
22 dioxines, i.e. molecular graphs with the chemical formula
C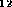 O
O H
H Cl
Cl and the prescribed skeleton
like in fig.
and the prescribed skeleton
like in fig.  , and so on.
, and so on.
Figure 2: Skeleton of dioxine
Some of these isomers are shown in many textbooks, too.
Further numbers and CPU times (with respect to a 486 DX2/66 running
OS/2) are gathered in the following table:
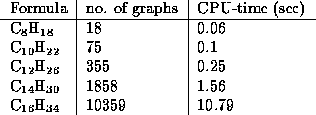
These numbers of isomers agree with the numbers obtained by ESESOC
(cf. [11]), but the present generator seems to be more efficient. In
general the numbers of isomers grow very rapidly,
as shown in table 2.



Next: The use of
Up: MOLGEN, a generator of
...
Previous: The computation of
Send questions to: molgen@btm2x2.mat.uni-bayreuth.de
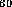 , prescribes valence 3 for every carbon
atom (since in fact C
, prescribes valence 3 for every carbon
atom (since in fact C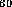 H
H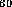 is meant), together with 12 fivemembered
rings of carbon atoms as macro-atoms, the generator yields 7848 simple graphs
consisting of 12 points (=macro-atoms) of valence 5. In the next step of
expanding the macro-atoms
MOLGEN starts generation (since the
total number of atoms is less than 100), but the generated number of molecular
graphs will be more or less infinite, even if there are 10
nonoverlapping benzene rings prescribed as macroatoms.
More moderate questions show that there are
altogether 217 molecular graphs for C
is meant), together with 12 fivemembered
rings of carbon atoms as macro-atoms, the generator yields 7848 simple graphs
consisting of 12 points (=macro-atoms) of valence 5. In the next step of
expanding the macro-atoms
MOLGEN starts generation (since the
total number of atoms is less than 100), but the generated number of molecular
graphs will be more or less infinite, even if there are 10
nonoverlapping benzene rings prescribed as macroatoms.
More moderate questions show that there are
altogether 217 molecular graphs for C H
H that there are exactly
22 dioxines, i.e. molecular graphs with the chemical formula
C
that there are exactly
22 dioxines, i.e. molecular graphs with the chemical formula
C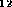 O
O H
H Cl
Cl and the prescribed skeleton
like in fig.
and the prescribed skeleton
like in fig.