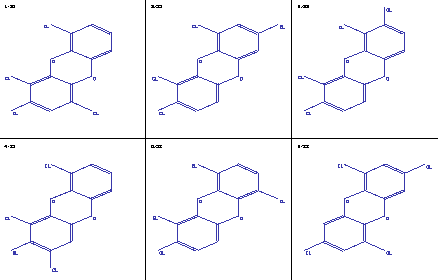
Figure 1: Molecule tapestry of dioxin isomers
After the generation of the constitutional isomers, you get a sketch of the molecules using a method based on [6], either in form of a tapestry, where several molecules are shown together on the screen, usually 6 of them, or you can get a single molecule on the screen.

Figure 1: Molecule tapestry of dioxin isomers
Fig. 1 shows the printed version of a tapestry of dioxin isomers.
The next step is the computation of a placement of that constitutional isomer in space. This is done by an application of the (simplified) MM2-model of Allinger (see. [7]) using an optimization programme. You can watch the result on the screen, rotate it, color it, replace the atoms by little balls, and so on.
Each of the structural isomers may exist in several configurations in space. MOLGEN is capable of generating all possible configurational isomers, again redundancy free (which, of course, also implies the consideration of symmetries).
The notion stereo isomerism is not uniquely defined in chemistry; it should therefore be stated which kinds of effects are taken into account. Primarily there are:
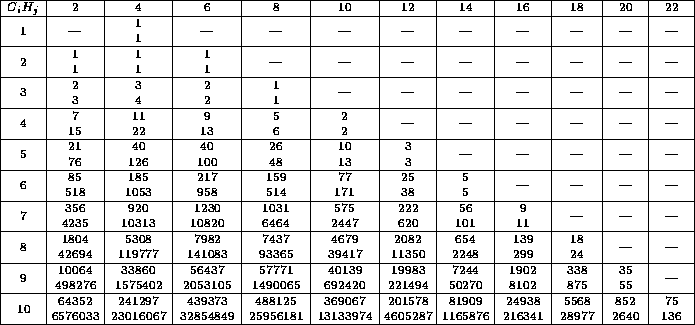
Table: Each entry consists of the number of constitutional isomers
as in table 1, repeated for the convenience of the reader,
and the number of configurational isomers, arising from the first.
The row index 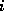 denotes the number of C-atoms, the column index
denotes the number of C-atoms, the column index
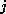 the number of H-atoms.
the number of H-atoms.
In the second step spatial realizations of these isomers are calculated
by the application of appropriate geometrical transformations to
the placement computed above.
(The basics of these calculations, which are again discussed in
[9], can be found in [10].)
In fig. ![]() the four stereoisomers of 1,2,3,4-tetramethylcyclobutane
are displayed as an example.
the four stereoisomers of 1,2,3,4-tetramethylcyclobutane
are displayed as an example.
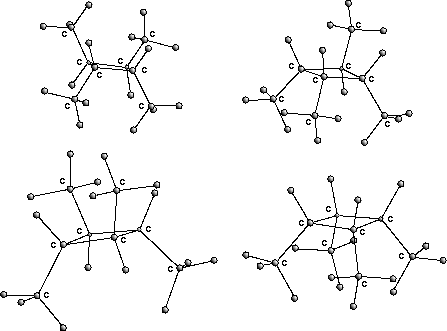
Figure: stereoisomers of 1,2,3,4-tetramethylcyclobutane
The cycle structure of the molecule and the position of the stereo-centers
sometimes make an accurate transformation impossible. In this case,
approximate constructions are performed which
can be optimized afterwards.
Send questions to: molgen@btm2x2.mat.uni-bayreuth.de